|
 Instructions For Use Instructions For Use
|
What Is  iPPFSS?
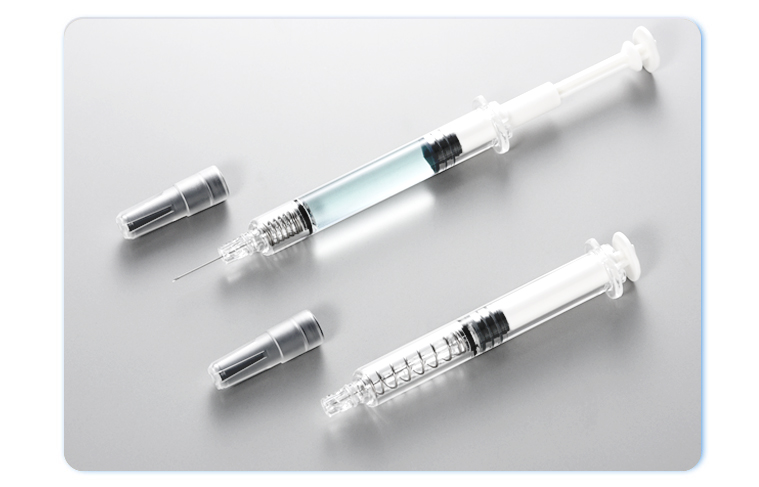
 iPPFSS iPPFSS is the short name of  Integrated Plastic Pre-fillable Safety Syringe Integrated Plastic Pre-fillable Safety Syringe. It is also known as  PFS PFS, or  Syringe Syringe, or  . Designed and developed by MedicalChain International Corp.(MCI), it is sterile, single-use, pre-fillable, one-handed operable with visible and audible active needle retraction safety features to reduce the risk of sharp injuries after injection. The material
of its container closure is made of COP (Cyclo-olefin Polymer) without breakage problem as traditional glass pre-filled syringe. The packaging of  is in the ready-to-fill nest & tub format compatible with conventional drug fill / finish process and equipment.
The Trend of PFS
Rapid growth and increased competition in the biotechnology industry are driving the need to differentiate among products. Improvements in product presentation form, evolving from vials to pre-filled syringes (PFS), and other quality attributes are key strategic factors one must consider in order to succeed in an increasingly competitive marketplace. Prefilled syringes offer several advantages for drugs requiring parenteral injection. On one hand, pharmaceutical companies can benefit from reduced cost of goods by eliminating overfill that are required by traditional packaging of vials/ampoules. On the other hand, patients and healthcare workers can benefit from a dosage form that is ready-to-use, improved dosing accuracy, and reduced risks associated with filling and using a syringe. Importantly, all these benefits can reduce overall healthcare cost by improving patient compliance.
In the years 2005 through 2007, unit sales of PFS have risen about 22% (Ref.1). The worldwide market for PFS has increased to an estimated 2.7B units in 2010 and the total revenues in this market are predicted to reach $3.9B in 2015, $4.6B in 2018 and $5.2B in 2021 (Ref. 2, 3). The growth in PFS market along with the adoption of the needle-stick safety and prevention laws worldwide have stimulated the Needle Safety Device (NSD) market in the past decade. However, despite its growing popularity, development of PFS as a method of drug delivery also has its own hurdles and risks.
Prefilled syringes act both as drug delivery vehicle and the primary container for drug products. Therefore, they are required to provide seal integrity, drug compatibility and stability throughout the shelf life of the drug product. Glass, the traditional material of construction for PFS has also been challenged. There have been cases of huge financial loss and damaged corporate reputation in the pharmaceutical industry associated with recalls of valuable combined products due to glass breakage (Ref 4). Apart from its heavy and brittle nature, residual tungsten in the glass barrel from the bore opening process can cause protein degradation. Drug stability under high pH value also presents another risks associated with glass barrel.
Furthermore, prefilled syringes currently available on the market do not have integrated needle-stick prevention function; they all require certain add-on sheaths or other devices to render the syringe sharp safety, retrofitting of said external device onto the glass barrel also comes with additional risks of subjecting an expensive drug through another assembly process.
Ref:
- http://www.ondrugdelivery.com/publications/Prefilled_syringes_%2007.pdf
- PREFILLED SYRINGES: Drugs, Devices and Disease Therapeutics (Greystone report, May 2009)
- Pre-Filled Syringes: World Market Outlook 2011-2021 (Visiongain report)
- http://www.fda.gov/Safety/Recalls/EnforcementReports/ucm247463.htm
MCI’s Solution to Unmet Needs
In order to address the unmet need for a PFS that is break-resistant, free of heavy metal, and features an integrated safety system, MCI is developing an innovative Integrated Plastic Pre-fillable Safety Syringe (iPPFSS), with brand name  as mentioned above, made of COC or COP polymer. The design is fully integrated with MCI’s patented user-controlled automatic needle-retracting mechanism which has been proven in automated mass production of safety syringes for clinical use. Although the same technology platform is employed for prefillable version, certain configurations have been fine-tuned to enhance container closure integrity. Furthermore, the dimensions of the prefillable syringes are adjusted to accommodate conventional ready-to-use fill/finish system as well as sterilization process so that manufacturing switching cost is eliminated or minimized. as mentioned above, made of COC or COP polymer. The design is fully integrated with MCI’s patented user-controlled automatic needle-retracting mechanism which has been proven in automated mass production of safety syringes for clinical use. Although the same technology platform is employed for prefillable version, certain configurations have been fine-tuned to enhance container closure integrity. Furthermore, the dimensions of the prefillable syringes are adjusted to accommodate conventional ready-to-use fill/finish system as well as sterilization process so that manufacturing switching cost is eliminated or minimized.
In order to shorten the time required for product development and commercialization, standard and FDA compliant products from leading suppliers will be used for all the components that in contact with the active pharmaceutical ingredient, such as plunger (stopper or piston) and needle shield.
 iPPFSS takes advantage of innovative material design and manufacturing flexibility, eliminates glass breakage and provides a great life cycle management tool for drug products in PFS. It could ensure the quality of syringes before fill and reduces waste of precious drugs due to added assembly process which is occasionally needed with the add-on devices. Its compact design saves costs in cold storage, shipping, handling (lower density/weight) and waste disposal. iPPFSS takes advantage of innovative material design and manufacturing flexibility, eliminates glass breakage and provides a great life cycle management tool for drug products in PFS. It could ensure the quality of syringes before fill and reduces waste of precious drugs due to added assembly process which is occasionally needed with the add-on devices. Its compact design saves costs in cold storage, shipping, handling (lower density/weight) and waste disposal.
 iPPFSS’s Advantages
 iPPFSS Advantages: iPPFSS Advantages:
- Patented Technologies & Freedom to Operate
- Integrated Safety Feature Activated by One Hand
- No Additional Assembly after Drug Fill / Finish
- Compatible with Standard F/F Process & Equipment
- Made of COP and Biocompatible Wetted Components
- Glass Like Transparency and High Break Resistance
- High pH Tolerance, No pH-Shift
- Low Extractables and Leachables
- Proven Standard Plunger and Needle Shield
- Ease-of-Use and Time Saving with 3 Simple Steps
- Cost Saving in Shipping, Cold Storage, Waste Disposal
- Competitive Price vs Glass PFS Plus Safety Device
Preliminary Product Specifications of 
PRELIMINARY PRODUCT SPECIFICATION
-
GENERAL DESCRIPTION
The  Integrated Plastic Pre-fillable Safety Syringe, in short Integrated Plastic Pre-fillable Safety Syringe, in short  iPPFSS, or iPPFSS, or  PFS, or PFS, or  Syringe, or Syringe, or  , design and developed by MedicalChain International Corp. ( MCI), is sterile, single-use, pre-fillable, one-handed operable with visible and audible active needle retraction safety features to reduce the risk of sharp injuries after injection. The packaging of , design and developed by MedicalChain International Corp. ( MCI), is sterile, single-use, pre-fillable, one-handed operable with visible and audible active needle retraction safety features to reduce the risk of sharp injuries after injection. The packaging of  is in the ready-to-fill nest & tub format compatible with conventional drug fill / finish process. is in the ready-to-fill nest & tub format compatible with conventional drug fill / finish process.
 works like a conventional hypodermic syringe except for its ability to retract the contaminated needle into the syringe barrel by user activation after injection. The works like a conventional hypodermic syringe except for its ability to retract the contaminated needle into the syringe barrel by user activation after injection. The  consists of the following typical components. consists of the following typical components.
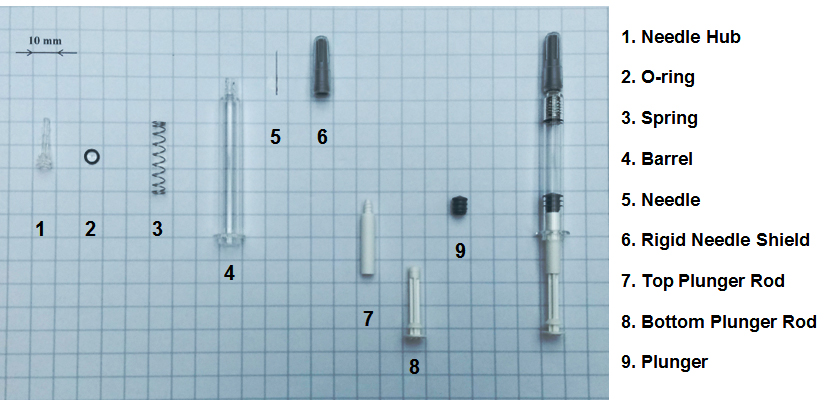
Except for spring, top plunger rod and bottom plunger rod, other components contacted with drug solution are all in compliance with the USP, EP, JP and ISO standards for pharmaceutical industry. The needle, rigid needle shield, O-ring, spring and plunger are purchased from existing pre-filled syringe (PFS) suppliers and directly used in  without further processing. The injected parts of needle hub, barrel, top plunger rod, and bottom plunger rod are contract manufactured by qualified suppliers. The suppliers of the rigid needle shield, plunger and COP material of needle hub and barrel had been assigned the Drug Master File (DMF) Number by US FDA for their formulations or products to meet the pharmaceutical regulatory requirements. All the necessary tests are under conducting according to relevant regulations for longer aging time. The Extractable/Leachable study reports will be provided under MNDA. There are drug companies of heparin, vaccine, and biologics working with MCI for Drug-Device Stability Study. without further processing. The injected parts of needle hub, barrel, top plunger rod, and bottom plunger rod are contract manufactured by qualified suppliers. The suppliers of the rigid needle shield, plunger and COP material of needle hub and barrel had been assigned the Drug Master File (DMF) Number by US FDA for their formulations or products to meet the pharmaceutical regulatory requirements. All the necessary tests are under conducting according to relevant regulations for longer aging time. The Extractable/Leachable study reports will be provided under MNDA. There are drug companies of heparin, vaccine, and biologics working with MCI for Drug-Device Stability Study.
- Physiochemical Properties
| No. |
Test Name |
Standard |
Specification |
Test Result |
| Typical Value |
| 1 |
Appearance of Solution |
USP <661.2> |
Clear and Colorless |
Clear and colorless |
Pass |
| 2 |
Absorbance |
USP <661.2> |
Amax 230-360nm ≦0.2 |
0.09 |
Pass |
| 3 |
Acidity or Alkalinity |
USP <661.2> |
Colorless / Pink / Orange-Red |
Colorless / Pink / Orange-Red |
Pass |
| 4 |
Total Terephthaloyl Moieties, PET, and PETG |
USP <661.2> |
A 244 nm of 50% alcohol extract ≦ 0.15 |
0.11 |
Pass |
| 5 |
Elemental Leachables Analysis with ICP-MS |
USP <1664> |
Sb, As, Cd, Cu, Pb, Li, Ni, V, Zn, Al, Cr, Ti, Zr, W ≦50ppb |
Lower |
Pass |
| 6 |
Semivolatile Organic Leachables Analysis with GC-MS |
USP <1664> |
lower than AET(0.15 µg/mL, for the highest-risk drug) |
Lower |
Pass |
- Physical Properties
| No. |
Test Name |
Standard |
Specification |
Test Result |
| Typical Value |
| 1 |
Break Loose Forces Test |
ISO 11040-8 : 2016 |
Agreement between Manufacturer & Customer |
≦816 gf |
Pass |
| ISO 7886-1 : 2017 |
| Internal SIP |
≦1500 gf |
| 2 |
Glide Force Test |
ISO 11040-6 : 2012 |
Average≦ 500 gf |
147~ 304 gf |
Pass |
| ISO 7886-1 : 2017 |
| Internal SIP N-04-04-02 |
≦ 500 gf |
| 3 |
Needle Hub Push-Out Force |
Internal SIP N-04-04-03 |
1.50~3.50Kgf |
1.908~2.91Kgf |
Pass |
| 4 |
Plunger Rod Collapsed Force Test |
Internal SIP N-04-04-04 |
4.00~6.00 Kgf |
4.242~4.987 Kgf |
Pass |
| 5 |
Safety Activation Force Test by Hand |
Internal SIP N-04-04-05 |
Needle Retracted Completely |
Needle Retracted Completely |
Pass |
| 6 |
Needle Penetration Force Test |
ISO 11040-6 : 2012; ISO 7864: 2016 |
Agreement between Manufacturer & Customer |
63~103 gf |
Pass |
| Internal SIP N-04-04-06 |
≦ 110 gf |
| 7 |
Needle Pull-Out Force Test |
ISO 11040-6 : 2012 |
|
≧2.204 Kgf |
Pass |
| ISO 7864: 2016 |
≧1.50 Kgf |
| 8 |
Closure System Liquid Leakage Test |
ISO 11040-6 : 2012 |
No tip cap falls, or no droplets visible |
No Leakage |
Pass |
| ISO 7886-1 : 2017 |
| 9 |
Plunger Liquid Leakage Test |
ISO 11040-8 : 2016 |
No leakage through plunger |
No Leakage |
Pass |
| ISO 7886-1 : 2017 |
| 10 |
Needle Shield Pull-Off Force Tset |
ISO 11040-6 : 2012 ; ISO 7886-1 : 2017 |
Agreement between Manufacturer & Customer |
503~1793 gf |
Pass |
| Internal SIP N-04-04-10 |
300 gf ≦X≦2000 gf |
| 11 |
Dead Space |
ISO 11040-6 : 2012 |
≦0.07 mL |
0.00794~0.00887 mL |
Pass |
| ISO 7886-1: 2017 |
| 12 |
Lubricant & Distribution |
ISO 11040-6 : 2012 |
Agreement between Manufacturer & Customer |
0.10±0.05 mg |
Pass |
| Internal SIP N-04-04-12 |
≦ 0.4 mg |
| 13 |
Particulates Test |
ISO 11040-6 : 2012 |
|
|
Pass |
| USP <788> |
Particles≧10µm:600 max/mL |
15~53 / mL |
| Particles≧25µm: 60 max/mL |
2~4 / mL |
| 14 |
Flange Breakage Resistance Test |
ISO/DIS 11040-6 : 2017 |
Agreement between Manufacturer & Customer |
≧ 48 Kgf |
Pass |
| Internal SIP N-04-04-14 |
≧ 30 Kgf |
| 15 |
Luer Cone Breakage Resistance Test |
ISO/DIS 11040-6 : 2017 |
As Above |
No Inclination |
Pass |
| Internal SIP N-04-04-15 |
Inclination Angle ≦ 0.15° dropped from 1M height |
| 16 |
Permeation Test |
USP 671 |
Water weight loss does not exceed 2.5%/ yr in not more than 1 of the 10 test container and does not exceed 5.0%/yr in any of them. |
0.26~1.84 % |
Pass |
| 17 |
Container Closure Integrity Test (CCI) |
ISO 11040-6 : 2012 |
Using dye solution tightness method |
No Leakage |
Pass |
- SHELF LIFE : The shelf life of
 is 2 years. is 2 years.
- STERILIZATION : The Nest-Tub Format packaged
 is sterilized by EtO. is sterilized by EtO.
- OTHER RELEVANT TESTS ARE UNDER CONDUCTING.
Stability of Drug Products in  and Glass PFS under Shipping Stress
Introduction
Pre-filled syringe (PFS) has become an attractive container closure option due to cost savings and improved patient acceptance. It is known that compatibility between drug formulation and container closure is a regulatory requirement due to the influence drug stability has on its safety and efficacy. The content and morphology of Sub-Visible Particle (SbVP, size range from 2 to 100 mm) in drug product is a sensitive indicator of stability and an important quality parameter that has gained increased attention from regulatory bodies in both US and EU, due to its link to product safety.
The purpose of this study is to compare stability of two different pharmaceutical molecules in two types of PFS – a traditional glass PFS and a novel PFS that is made of polymer with a integrated, built-in, safety feature to prevent needle stick injury named  . To achieve this objective, Leukocyte Growth Factor (LGF) Biosimilar and Enoxaparin Sodium (ES) solutions were subjected to accelerated stress testing by agitation in order to simulate the stress that occurs due to routine shipping. Agitation can lead to drug instability, especially protein pharmaceuticals, by exposing the molecules to air-liquid interface, which leads to protein unfolding and subsequent aggregation. Dynamic Image Analysis (FlowCAM), a sensitive method capable of obtaining quantitative and morphological information, was used for real-time monitoring of SbVPs in these drug products. . To achieve this objective, Leukocyte Growth Factor (LGF) Biosimilar and Enoxaparin Sodium (ES) solutions were subjected to accelerated stress testing by agitation in order to simulate the stress that occurs due to routine shipping. Agitation can lead to drug instability, especially protein pharmaceuticals, by exposing the molecules to air-liquid interface, which leads to protein unfolding and subsequent aggregation. Dynamic Image Analysis (FlowCAM), a sensitive method capable of obtaining quantitative and morphological information, was used for real-time monitoring of SbVPs in these drug products.
Material and Methods
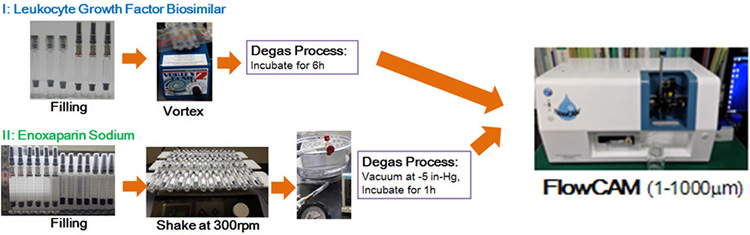
Filling of LGF and ES Solution into  and Glass PFS and Glass PFS
- Remove the needle cover, assemble the plunger with plunger rod, insert plunger into the end part of the barrel, and purge air from syringe.
- Insert the syringe needle into the solution surface and pull the plunger back to 1ml mark.
- Straighten the syringe with the needle upward and gently tap the barrel to move air-bubble to the top of meniscus.
- Gently pull the plunger and keep the 2-3mm air-gap in the syringe barrel.
- Assemble the needle cover and syringe.
Agitation and Degassing Processes For LGF drug product
- PFS were fixed on the plate of Vortex-Genie apparatus and vortexed for 0-60min.
- Following agitation PFS were allowed to stand for 6 hours to remove small air-bubbles from solution.
For ES drug product
- PFS were fixed on the plate of orbital shaker apparatus and shaken at 300 rpm for
0-3 days.
- Following agitation PFS were placed in a desiccator, vacuumed at -5 in-Hg for 60 min, and allowed to stand for 60min to remove air-bubbles from solution.
Results and Discussion
Drug Product Study I: Stability of LGF Biosimilar in Glass and  PFS Following Exposure to Vortex Stress PFS Following Exposure to Vortex Stress
Some irregularly shaped SbVPs were detected in the original LGF Biosimilar solution (in vial) (Fig. 1A), which is likely due to protein aggregates that are formed under sub-optimal formulation conditions. Following exposure to rigorous agitation stress, the concentration of Silicone-Oil (Si-Oil) SbVPs and Non-Silicone-Oil (Non-Si-Oil) SbVPs increased significantly in both types of PFS (Fig. 2). Based on morphological features of the particles, the round SbVPs are likely released from the silicone oil film coated on the inner wall of PFS, and the irregularly shaped SbVPs are mainly consisted of aggregated protein from LGF Biosimilar1-4. Compared to Glass PFS (Fig. 1B), the level of Si-Oil and Non-Si-Oil particulates found in LGF Biosimilar are approximately ten times lower in  PFS (Fig. 1C). Therefore, it can be concluded that LGF Biosimilar is likely to be more stable in  PFS than Glass PFS during routine shipping and handling.
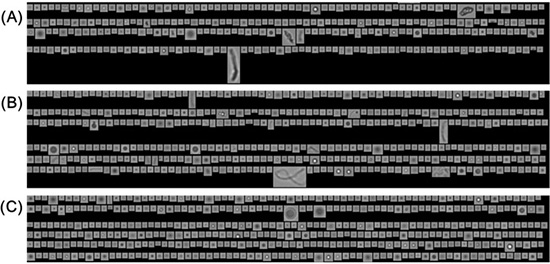
Fig. 1
The SbVP images of Leukocyte Growth Factor Biosimilar with vortex 60min.
(A) in vial
(B) in Glass PFS
(C) in  PFS PFS
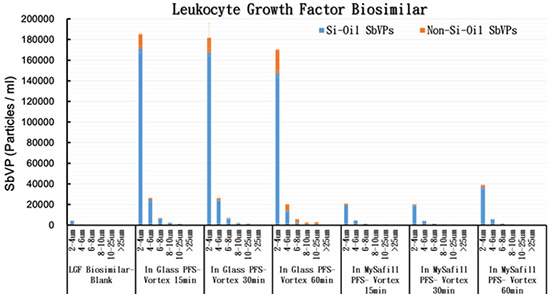
Fig. 2 The SbVP content of LGF Biosimilar in Glass PFS and  PFS
with vortex 15, 30 and 60 min. PFS
with vortex 15, 30 and 60 min.
Drug Product Study II: Stability of Enoxaparin Sodium in Glass and  PFS Following Exposure to Shaking Stress PFS Following Exposure to Shaking Stress
Initially there were a few small black SbVPs, most likely air-bubbles, in the ES solution (in vial, Fig. 3A). Following shaking at 300rpm for 3 days, most SbVPs of ES found in PFS are small white round Si-Oil particles that were released from inner wall of PFS (Fig. 4). Compared with both types Glass PFS (type 1 and type 2), fewer SbVPs were formed in  PFS (Fig. 4), and there were no Non-Si-Oil type SbVP in PFS (Fig. 4), and there were no Non-Si-Oil type SbVP in  PFS (Fig. 3B). These data suggest that PFS (Fig. 3B). These data suggest that  PFS is superior to glass PFS for maintaining stability of ES under shipping stress. PFS is superior to glass PFS for maintaining stability of ES under shipping stress.

Fig. 3 The SbVP images of Enoxaparin Sodium
with shaking at 300 rpm for 3days.
(A) in vial
(B) in 
(C) in Type 1 Glass
(D) in Type 2 Glass
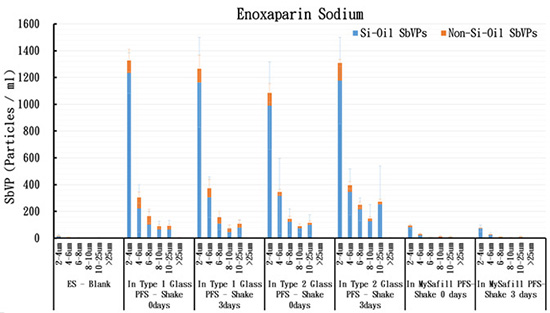
Fig. 4
The SbVP content of Enoxaparin Sodium in
Glass PFS and
 with shaking at 300 rpm for 0, 3 days. with shaking at 300 rpm for 0, 3 days.
Conclusion
Under rigorous physical stress, the Si-Oil and Non-Si-Oil SbVP contents of a protein and polysaccharide drugs in  PFS are fewer than in traditional Glass PFS. Given that SbVP level is a highly sensitive indicator of a molecule’s physical stability, our data from mechanical stress (agitation) testing suggest that during routine shipping Enoxaparin Sodium and LGF Biosimilar will likely be more stable in PFS are fewer than in traditional Glass PFS. Given that SbVP level is a highly sensitive indicator of a molecule’s physical stability, our data from mechanical stress (agitation) testing suggest that during routine shipping Enoxaparin Sodium and LGF Biosimilar will likely be more stable in  PFS than in Glass PFS. PFS than in Glass PFS.
 Advantages
- Patented Technologies & Freedom to Operate
- Integrated Safety Feature Activated by One Hand
- No Additional Assembly after Drug Fill / Finish
- Compatible with Standard F/F Process & Equipment
- Made of COP and Biocompatible Wetted Components
- Glass Like Transparency and High Break Resistance
- High pH Tolerance, No pH-Shift
- Low Extractables and Leachables
- Proven Standard Plunger and Needle Shield
- Ease-of-Use and Time Saving with 3 Simple Steps
- Cost Saving in Shipping, Cold Storage, Waste Disposal
- Competitive Price vs Glass PFS Plus Safety Device
Reference
-
Rosenberg AS. 2006. Effects of protein aggregates: An immunologic perspective. AAPS J 8:E501–E508.
- Shankar G, Shores E, Wagner C, Mire-Sluis A. 2006. Scientific and regulatory considerations on the immunogenicity of biologics. Trends Biotechnol 24:274–280.
- Danny K. Chou, 2014, SCPDG Meeting, Protein Aggregation and Emerging Tools to Support Development and Characterization.
- Mahler H, Friess W, Grauschopf U, Kiese S. 2009. Protein aggregation: Pathways, induction factors, and analysis. J Pharm Sci 98:2909–3934.
Invite Drug Companies to join  for Drug-Device Compatibility / Stability Study
|